Common Disease, Uncommon Approach
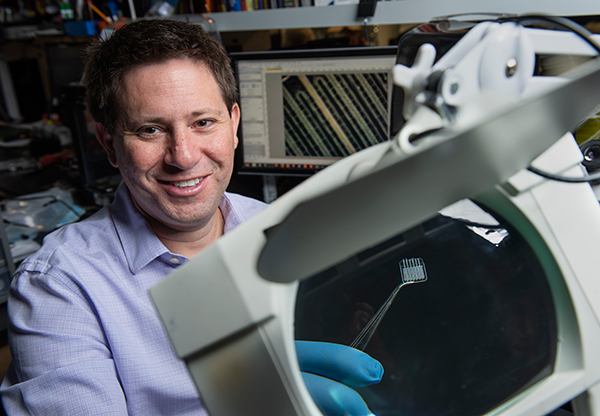
Aug. 1, 2018 - When Samueli School doctoral candidate Rachel Gurlin was 11 years old, her younger sister was diagnosed with Type 1 diabetes, and Gurlin watched as she injected herself several times each day with insulin to regulate her blood sugar. Now Gurlin, who works in the lab of biomedical engineering professor Elliot Botvinick, is helping create an implantable device that could enable her sister – and 20-40 million other Type 1 patients worldwide – to permanently eliminate difficult treatments.
Type 1 diabetes is an autoimmune disease. The body attacks beta cells in the pancreas, preventing the organ from producing the insulin needed to move glucose from the bloodstream into other cells. In addition to insulin shots, treatments include insulin pumps, which continuously deliver themedication through a catheter; or pancreasor islet transplantation, which canunleash a host of complications.
Gurlin and Botvinick’s small device functions as abioartificial pancreas, using atwo-step process to deliverinsulin naturally. First,the device is implantedsubcutaneously, possiblyin the lower back. Afterimplantation, blood vesselsfrom the host tissue grow intoslits within the device, providingoxygenation to the local area. Inthe second step, islets (clusters ofhormone-producing cells) are inserted intothe implanted device. Those islets could comprisedonor cells or stem cells cultivated to become islets, but they will interact with the tissue around them while producing insulin – just like the pancreas does.
Gurlin and Botvinick call their two-step procedure a “civil engineering” approach. “We build the house first, put in the plumbing (blood vessel growth and tissue interaction), inspect it, and then populate it (with the islets),” says Gurlin.Measuring 13 millimeters square in its current iteration for testing in mice, the device will scale up for humans to about the size of a business card. It is made of medical grade silicone, which allows oxygen to pass though, and its one-millimeter depth means multiple layers can be stacked on top of one another to increase the number of islets housed and the amount of insulin produced.
Gurlin makes plastic molds in a 3D printer, fills them with liquid silicone and bakes them before removing the devices, each of which has multiple tiny channels within for holding the islets. After the patient’s tissue grows successfully into the implanted device, islets are injected using long microtubing connected to a syringe. Researchers hope the islets and the patient’s tissue will mutually support each other.
There are two major hurdles: keeping tissue oxygenated and protecting the implanted device from immune system rejection. The slits built into the flexible silicone seem to be meeting the first challenge by allowing the recipient’s own tissue to grow and thrive in the device.
The second challenge ismore difficult. An implanted device triggers two immune responses: inflammation, as thebody surrounds the invader withscar tissue; and an adaptive reaction that creates antibodies to destroy transplanted cells.
Gurlin and Botvinick are working closelywith Eugenia Kharlampieva, associate professor of polymer chemistry at University of Alabama at Birmingham, whose lab is developing an ultrathin anti-inflammatory coating to prevent scar tissue from forming. Made from natural compounds, the coating can be deposited directly on the device surface to prevent the recipient’s immune system from rejecting the implant.
“I am very excited about this collaboration as this is a wonderful opportunity to apply our material to these devices,” Kharlampieva says. “We hope our coating will be able to suppress undesirable immune responses to ensure successful implantation.”
The adaptive immune system is more difficult. A number of approaches are under investigation, and Gurlin and Botvinick intend to be ready. “Rachel is staying ahead of the game. As the biologists develop [these], she’s making sure there is a device ready for them,” says her mentor, who credits Gurlin with overcoming multiple obstacles. “There are so many difficult elements to this, and in every challenge, she has created breakthroughs.”
Testing in mice is underway but researchers are wary of predicting the onset of human testing. “Type 1 patients have been promised time and time again that a cure is around the corner,” Gurlin says. “Out of respect for them, we want to work as hard as possible … but will not promise any timelines until we are sure.”
Regardless, Botvinick is optimistic about long-term success. “This project is a huge challenge in any context, and we don’t shy away from that,” he says.
“I feel that tissue therapy will be the ultimate cure for diabetes. And by cure,” he adds, “I mean a complete biological reversal of the disease. You don’t have to do anything to manage it; you don’t have to take pills or poke yourself or monitor your sugar. It’s complete autonomous control by cells.”
- Anna Lynn Spitzer
