Materials Science Transforms Defects into Opportunity
May 13, 2019 - All materials contain defects due to the way atoms interact while they are assembling. Materials scientists seek ways to reduce these defects or, in some cases, even use them to the material’s advantage.
In a paper published this spring in the journal Physical Review Letters, Samueli School researchers detail findings that could turn certain defects into benefits, providing materials with increased strength, toughness, resistance to fatigue or other important characteristics.
The paper’s first author, postdoctoral researcher Vladyslav Turlo, along with Tim Rupert, associate professor of materials science and engineering, proved the existence of tiny nanoparticle arrays that form along dislocations inside the material. Dislocations are a type of linear defect that can move around inside the substance, resulting in permanent shape change known as plastic deformation. But contrary to what the name implies, these linear defects actually can be engineered to improve the material.
“These dislocations turn out to be the number one thing that controls the strength of a material,” Rupert says. “Deformation is not always bad, not always something you want to avoid, but it’s something you want to understand and even perhaps learn how to control. This is a new idea where we’re trying to control the local structure of these important defects.”
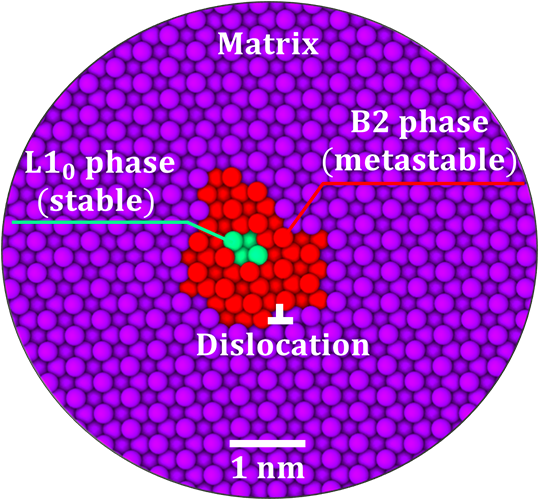
Using atomistic computer simulations, Turlo and Rupert discovered that unique kinds of nanoparticles exist along these defects in an iron-nickel alloy. In combination, the nanoparticles and the defects are known as linear complexions. The nanoparticles could be compared to a hard candy with a soft center; the outside ring contains atoms in one phase that is “metastable” or not expected based on what is known about the bulk materials, while inside, the atoms exist as a different phase. While the inner core structure was discovered recently by another experimental study, the metastable layer of the particles had been missed, but is essential for understanding how the particles form.
The nanoscale metastable phase is impossible to see with an optical microscope and nearly impossible to see even with an advanced electron microscope. But computer simulations of the defects definitively confirm their existence and give researchers a new understanding of how they form and how to control them.
Researchers can use the thermodynamic information obtained in Turlo and Rupert’s study to change the conditions of these linear complexions in order to alter the material’s response. Options include varying the composition of material as well as the amount of heat applied during processing to see how these changes affect the behavior of the defects.
“What is kind of beautiful about this is that we used nucleation theories that are a hundred years old, because this is just how phases behave in solids,” Rupert says. “But we were able to add a much more nuanced description of the area around the dislocation that you couldn’t get before,” allowing for improved understanding of the composition, heat and other factors that cause these linear complexions to form.
Now that they have proven these nanoparticles exist in iron nickel, Rupert and Turlo are working to extend their research to other material classes: nickel aluminum, aluminum zirconium, copper zirconium and aluminum copper, for starters. The nougat-type nanoparticles found in iron nickel are just one type of linear complexion, and Rupert says they expect to learn more about additional types of structures. “We are trying to understand all the different types of linear complexions and for each type, why they form and what the important processing conditions are,” he says. “Once we have these results, we want to test different hypotheses and make these novel materials a reality.”
This knowledge can lead to design of new materials for specific applications. These include structural materials that could be used to build stronger, more ductile and less fatigable buildings and bridges, as well as those that control diffusion, transport and other properties, which could be used for energy applications. “If you can understand how to control defects you can tune all types of properties,” Rupert says.
He adds that the new information also can be used to scale up production and processing of new materials. “You don’t have to make some tiny piece of material. If you know what processing conditions to use, you can do this with big chunks of material too.
“Our field is finally getting to the point where we can describe not just what happens after the fact ... but we can actually say, ‘I want this exact microstructure. I’m going to make this exact material and plan out exactly where my defects are.’ And that’s why we’re excited. This is a big step in the right direction.”
- Anna Lynn Spitzer
