“Just like the transistor is the basis of computers, the ion channel is the basic element in many processes in biology,” explains Peter Burke, who heads the nanotechnology group at the University of California at Irvine. He lists some of the aspects of the body they affect, which include neurons (and hence human thought) but also a variety of other processes, such as synthesis of the energy-storage molecule ATP in mitochondria. In fact, ion channels are crucial to so many physiological functions that they are the target of 25% of drugs produced, yet the technology for studying them using external current amplifiers has remained largely unchanged for 30 years. “Since we can manipulate and sense individual electrons in circuits, I thought it would be interesting to apply some of this knowledge in biology,” adds Burke.
Unsurprisingly, Burke and his colleagues are not the first to try to measure the currents of single ion channels. However, these measurements require a wide range of expertise covering both nanowires and physiology. Fortunately, Burke who holds multiple professorships at UCI was able to draw on his expertise in both these fields, as well as the extensive knowledge and experience of his collaborator Mark Reed from Yale University alongside co-authors Weiwei Zhou, LuyeMu, and Jinfeng Li.
“Previously people were looking at the action potential on a membrane, and treating the membrane like a capacitor;” says Burke. “This looks at a single channel so it’s a more difficult measurement.” Their nanowire device is the first to integrate the current measurement into the ion channel, which should ultimately allow much greater measurement sensitivity.
Sensing success
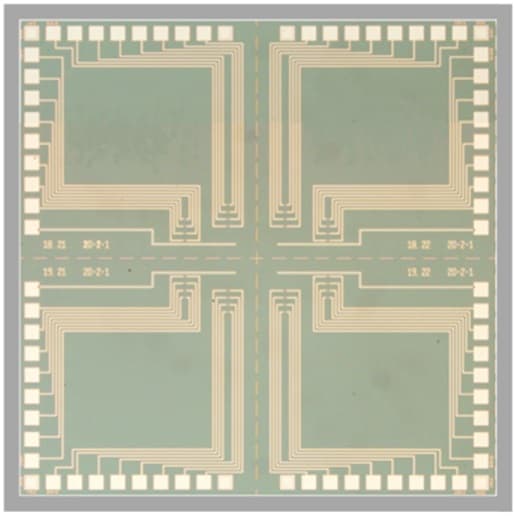
The researchers first demonstrated the ability to measure single ion channel currents using carbon-nanotube and graphene devices. However, Burke and his colleagues were keen to demonstrate the capability in a top-down fabricated silicon device that could leverage on the decades of development in that industry. This meant finding the right surface chemistry to attach the lipid bilayers, which act as artificial cell walls, to the silicon nanowire. They could then observe the behaviour of ion channel molecules on these bilayers.
They tested the device with two text book ion channels – alamethicin and gramicidin A. Both have antibiotic functions and work by making the bacterial membrane permeable. Ion channels responsible for vision and other neural functions have additional voltage and light dependencies and multiple states making observations of their behaviour more complicated. In their study the researchers observed the opening and closing of the gramicidin A ion channel and three of the four states in alamethicin.
The channels can open and close due to conformational changes in response to thermal fluctuations or interactions between peptides and the cell wall or by admitting some ions and blocking others. “One of the hopes of this work is to be able to apply this technology to answer in more detail: How do ion channels function?” says Burke.
While the behaviour of these ion channels was not affected by being integrated into the sensor, he points out that for larger ion channels that extrude into the space between the nanowire and membrane, this could be an issue. To tackle this the team have been able to demonstrate how to vary the space between nanowire and membrane with a tether.

Dynamic modelling
Different ion channels also have different opening times, leading to different “spike widths” in the observed current. “To be able to understand the dynamic behaviour of the ion channel itself we need to understand the behaviour of the ion channel within the circuit it makes with the sensing device,” Burke tells Physics World Materials. “We have a comprehensive circuit model that explains the connected system, which I call the ‘modified Hodgkin-Huxley model’.”
Alan Lloyd Hodgkin and Andrew Fielding Huxley won the Nobel Prize in Physiology and Medicine in 1963 for their mathematical description of how ionic transport gives rise to action potentials and allows them to propagate and carry signals through the neural system, enabling movement and other physical responses to sensory input. In the Hodgkin-Huxley model circuit elements represent different parts of the cell – for example a capacitor represents the lipid bilayer; a voltage- and time-dependent conductance represents the ion channel. Since the capacitance of the nanowire device is comparable to the lipid bilayer, Burke and co-authors could add this as another capacitance in the circuit model of the cell.
Next the team will look into scaling up the system to run millions of devices on a chip in parallel. They are also interested in measuring single ion channels from specific systems such as the heart, brain and other organs. Full details are reported in Nano Futures